Xience Sierra Stent
Bench test results may. The XIENCE Sierra stent system is indicated for improving coronary artery luminal diameter in patients including those with diabetes mellitus with symptomatic heart disease due to de novo native coronary artery lesions length 32 mm with reference vessel diameters of 225 mm to 425 mm.

The XIENCE V drug coated stent is used to treat coronary artery disease by propping open a narrowed or blocked artery and releasing the drug everolimus in a controlled manner to prevent the.

Xience sierra stent. The XIENCE Alpine stent systems are device drug combination products consisting of a drug-coated stent and a balloon expandable delivery system. Major bleeding BARC type 3 to 5 was less common in both XIENCE 90 22 vs 63 P for superiority 00001 and XIENCE 28 22 vs 45 P for superiority 00156 than in the XIENCE V cohort. It can be scanned safely under the conditions in the Instructions for Use.
The XIENCE Sierra stent system is indicated for improving coronary artery luminal diameter in patients including those with diabetes mellitus with symptomatic heart disease due to de novo native coronary artery lesions length 32 mm with reference vessel diameters of 225 mm to 425 mm. X The XIENCE V stent should be handled placed implanted and removed according to the Instructions for Use. It is unknown if the latest-generation durable polymer drug-eluting stent ie Xience Sierra would have resulted in a different outcome.
Hereafter the XIENCE Alpine family of stent systems is referred to as the XIENCE Alpine stent or XIENCE Alpine stent system. Therefore these findings are impressive. XIENCE Sierra continues to provide unparalleled safety 1 along with enhanced deliverability for even the most complex percutaneous coronary.
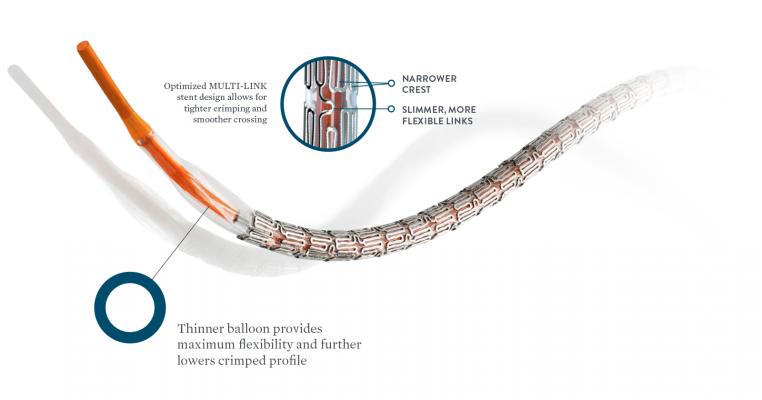
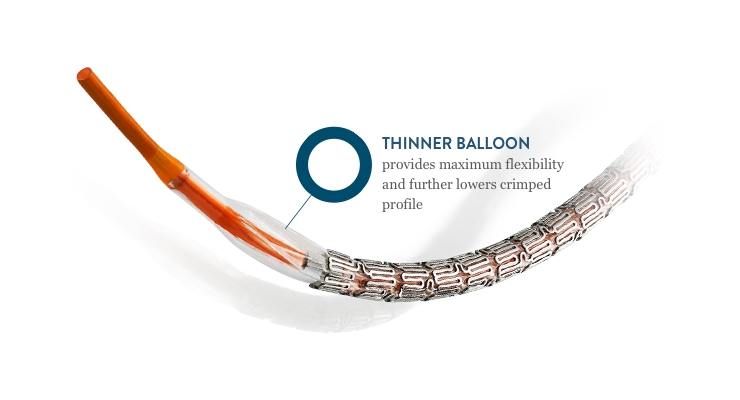
XIENCE Sierra 20 HIGHER IS BETTER Best-in-Class Deliverability1 Outstanding stent retention2 for confident crossing of challenging anatomy 1. And finally in XIENCE 90 ARC-defined definiteprobable stent thrombosis occurred at a rate of 020 between 3 and 12 months. The newest DES in the XIENCE family XIENCE Sierra Stent has an enhanced design that offers exceptional deliverability pushability post dilatation expansion as well as an ultra-low profile and smoother crossing.
1 The XIENCE safety heritage is now further strengthened with XIENCE Sierras exceptional acute performance. XIENCE Sierra everolimus-eluting coronary stent carries all the positive features of the precursor stents of the XIENCE family and brings an ultra-low crossing profile which further increases deliverability and flexibility. The stent is coated with a formulation containing everolimus the active ingredient embedded in a non-erodible polymer which is identical to the FDA approved XIENCE.
Randomized multicenter clinical trials reveal the efficacy and safety of using XIENCE Stents even in complex percutaneous coronary interventions PCI. Bench test data shows that XIENCE Sierra performed better in crossability and was not statistically different in trackability and pushability compared to Resolute Onyx and SYNERGY stents. As the worlds leading DES XIENCE Sierra Stent is the gold standard for safety in stent implantation.
Bench test data shows that XIENCE Sierra performed better in crossability and was not statistically different in trackability and pushability compared to Resolute Onyx and SYNERGY stents. XIENCE Sierra Drug Eluting Stent System. XIENCE Sierra Everolimus Eluting Coronary Stent System 40 x 18 mm n5 SYNERGY Stent System 40 x 20 mm n5 Resolute Onyx Stent System 45 x 18 mm n5.
Stent System hereinafter referred to as XIENCE V EECSS or XIENCE V stent system is a device drug combination product consisting of either the MULTI-LINK VISION Coronary Stent System or the MULTI-LINK MINI VISION Coronary Stent System coated with a formulation containing everolimus the active ingredient embedded in a non-erodible polymer. Xience sierra xience alpine xience family everolimus eluting coronary stent systems The XIENCE Family of Stents is indicated for improving coronary artery luminal diameter in patients including those with diabetes mellitus with symptomatic heart disease due to de novo native coronary artery lesions length 32 mm with reference vessel diameters of 225 mm to 425 mm. You know XIENCE as the worlds leading drug-eluting stent DES with an unparalleled safety record.
The comparator stent in this trial has a proven track record which makes it difficult for any experimental stent to show superiority with outcomes. The XIENCE Sierra stent system is indicated for improving coronary artery luminal diameter in patients including those with diabetes mellitus with symptomatic heart disease due to de novo native coronary artery lesions length 32 mm with reference vessel diameters of 225 mm to 425 mm. X Non-clinical testing has demonstrated that the XIENCE V stent in single and in overlapped configurations up to 68 mm in length is MR Conditional.
 Xience Sierra Stent Is Designed For Large Vessel Pci
Xience Sierra Stent Is Designed For Large Vessel Pci
 Abbott Gains European Approval For Xience Sierra Stent Daic
Abbott Gains European Approval For Xience Sierra Stent Daic
 About Xience Sierra Stent Crossability For Even Complex Cases
About Xience Sierra Stent Crossability For Even Complex Cases
 Xience Sierra Everolimus Eluting Coronary Stent System 2 75mm X 28mm Medical Materials
Xience Sierra Everolimus Eluting Coronary Stent System 2 75mm X 28mm Medical Materials
 Xience Sierra Everolimus Eluting Coronary Stent System
Xience Sierra Everolimus Eluting Coronary Stent System
 Xience Sierra Stent Stent Design
Xience Sierra Stent Stent Design
 Compare Drug Eluting Stents Stent Design And Clinical Data
Compare Drug Eluting Stents Stent Design And Clinical Data
 Fda Approves Abbott S Xience Sierra Everolimus Eluting Coronary Stent Medical Product Outsourcing
Fda Approves Abbott S Xience Sierra Everolimus Eluting Coronary Stent Medical Product Outsourcing
 A Evolution Of Outer Diameter With Pressure For Xience Sierra Endeavor Download Scientific Diagram
A Evolution Of Outer Diameter With Pressure For Xience Sierra Endeavor Download Scientific Diagram
 Abbott S New Xience Sierra Drug Eluting Coronary Stent Released In Europe Medgadget
Abbott S New Xience Sierra Drug Eluting Coronary Stent Released In Europe Medgadget
 Xience Sierra Everolimus Eluting Coronary Stent System
Xience Sierra Everolimus Eluting Coronary Stent System
 Abbott S Xience Sierra Stent Receives Fda Approval Daic
Abbott S Xience Sierra Stent Receives Fda Approval Daic
 Fda Oks Abbott S Next Gen Drug Eluting Stent Xience Sierra
Fda Oks Abbott S Next Gen Drug Eluting Stent Xience Sierra
 Xience Sierra Is Now On European Market Cardiovascular News
Xience Sierra Is Now On European Market Cardiovascular News
Comments
Post a Comment